Improved Synthesis and Mutagenicity of Oligonucleotides Containing 5-Hydroxymethylcytosine, 5-Formylcytosine and 5-Carboxylcytosine
02-Dec-2011
Chemistry, 2011, DOI: 10.1002/chem.201102782, Volume 17, Issue 49, pages 13782–13788 published on 02.12.2011
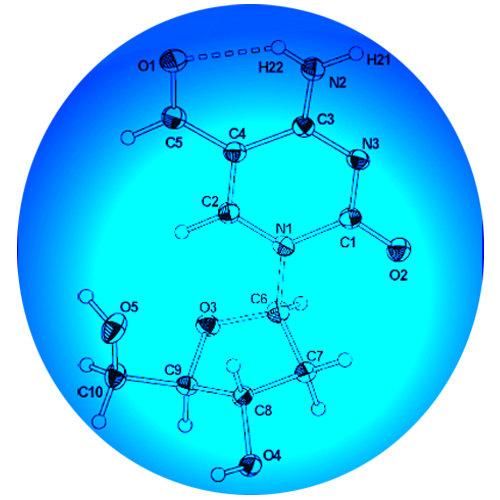
5-Formylcytosine (fC or 5-CHOdC) and 5-carboxylcytosine (caC or 5-COOHdC) have recently been identified as constituents of mammalian DNA. The nucleosides are formed from 5-methylcytosine (mC or 5-MedC) via 5-hydroxymethylcytosine (hmC or 5-HOMedC) and are possible intermediates of an active DNA demethylation process. Here we show efficient syntheses of phosphoramidites which enable the synthesis of DNA strands containing these cytosine modifications based on Pd0-catalyzed functionalization of 5-iododeoxycytidine. The first crystal structure of fC reveals the existence of an intramolecular H-bond between the exocyclic amine and the formyl group, which controls the conformation of the formyl substituent. Using a newly designed in vitro mutagenicity assay we show that fC and caC are only marginally mutagenic, which is a prerequisite for the bases to function as epigenetic control units.