Microsolvated and Chelated Butylzinc Cations: Formation, Relative Stability, and Unimolecular Gas-Phase Chemistry
23-Oct-2009
Chem. Eur. J., 2009, 15(46), 12745-12753, doi 10.1002/chem.200901963 published on 23.10.2009
Chem. Eur. J., online article
Chem. Eur. J., online article
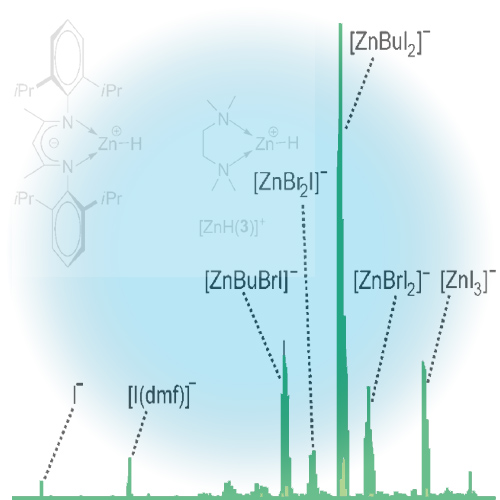
Solutions of butylzinc iodide in tetrahydrofuran, acetonitrile, and N,N-dimethylformamide were analyzed by electrospray ionization mass spectrometry. In all cases, microsolvated butylzinc cations [ZnBu(solvent)n]+, n=1-3, were detected. The parallel observation of the butylzincate anion [ZnBuI2]- suggests that these ions result from disproportionation of neutral butylzinc iodide in solution. In the presence of simple bidentate ligands (1,2-dimethoxyethane, N,N-dimethyl-2-methoxyethylamine, and N,N,N',N'-tetramethylethylenediamine), chelate complexes of the type [ZnBu(ligand)]+ form quite readily. The relative stabilities of these complexes were probed by competition experiments and analysis of their unimolecular gas-phase reactivity. Fragmentation of mass-selected [ZnBu(ligand)]+ leads to the elimination of butene and formation of [ZnH(ligand)]+. In marked contrast, the microsolvated cations [ZnBu(solvent)n]+ lose the attached solvent molecules upon gas-phase fragmentation to produce bare [ZnBu]+, which subsequently dissociates into [C4H9]+ and Zn. This difference in reactivity resembles the situation in organozinc solution chemistry, in which chelating ligands are needed to activate dialkylzinc compounds for the nucleophilic addition to aldehydes.