Molecular mechanisms behind the antimicrobial activity of hop iso-α-acids in Lactobacillus brevis
21-Oct-2015
Food Microbiology, Volume 46, Pages 553–563, doi:10.1016/j.fm.2014.09.017
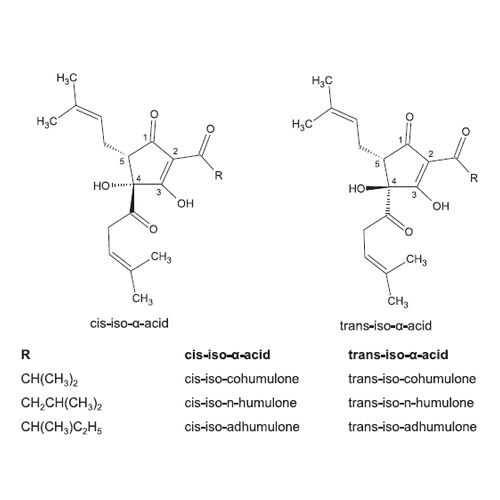
The main bittering component in beer, hop iso-α-acids, have been characterised as weak acids, which act as ionophores impairing microbial cells' function under acidic conditions as present in beer. Besides medium pH, divalent cations play a central role regarding the efficacy of the antimicrobial effect. The iso-α-acids' non-bitter derivatives humulinic acids can be found in isomerised hop extracts and can be generated during hop storage. Therefore, they have been under investigation concerning their influence on beer sensory properties. This study sketches the molecular mechanism behind iso-α-acids' antimicrobial activity in Lactobacillus (L.) brevis regarding their ionophore activity versus the dependence of the inhibitory potential on manganese binding, and suggests humulinic acids as novel tasteless food preservatives.
We designed and synthesised chemically modified iso-α-acids to enhance the basic understanding of the molecular mechanism of antimicrobial iso-α-acids. It could be observed that a manganese-binding dependent transmembrane redox reaction (oxidative stress) plays a crucial role in inhibition. Privation of an acidic hydroxyl group neither erased ionophore activity, nor did it entirely abolish antimicrobial activity. Humulinic acids proved to be highly inhibitory, even outperforming iso-α-acids.