Residue-Specific Side-Chain Packing Determines the Backbone Dynamics of Transmembrane Model Helices
20-Oct-2010
Biophysical Journal, 2010, doi:10.1016/j.bpj.2010.08.031, Volume 99, Issue 8, 2541-2549 published on 20.10.2010
Biophysical Journal, online article
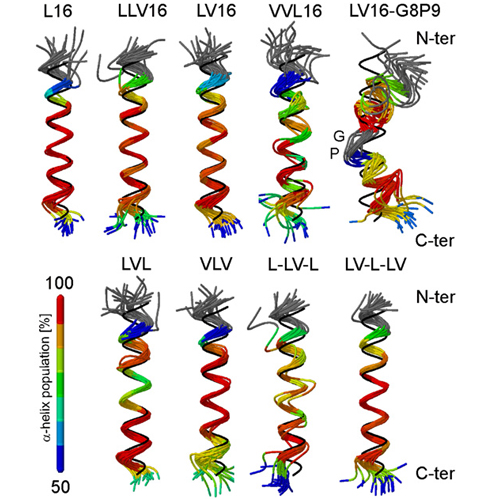
The transmembrane domains (TMDs) of membrane-fusogenic proteins contain an overabundance of b-branched residues. In a previous effort to systematically study the relation among valine content, fusogenicity, and helix dynamics, we developed model TMDs that we termed LV-peptides. The content and position of valine in LV-peptides determine their fusogenicity and backbone dynamics, as shown experimentally. Here, we analyze their conformational dynamics and the underlying molecular forces using molecular-dynamics simulations. Our study reveals that backbone dynamics is correlated with the efficiency of side-chain to side-chain van der Waals packing between consecutive turns of the helix. Leu side chains rapidly interconvert between two rotameric states, thus favoring contacts to its i plusminus 3 and i plusminus 4 neighbors. Stereochemical restraints acting on valine side chains in the alpha-helix force both b-substituents into an orientation where i,i plusminus 3 interactions are less favorable than i, plusminus 4 interactions, thus inducing a local packing deficiency at VV3 motifs. We provide a quantitative molecular model to explain the relationship among chain connectivity, side-chain mobility, and backbone flexibility. We expect that this mechanism also defines the backbone flexibility of natural TMDs.