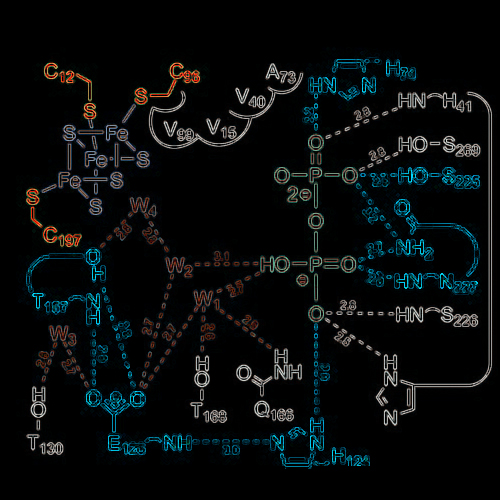
Structure of Active IspH Enzyme from Escherichia coli Provides Mechanistic Insights into Substrate Reduction
30-Jun-2009
Angewandte Chemie, 2009, Doi: 10.1002/anie.200900548, published on 30.06.2009
Angewandte Chemie, online article
Angewandte Chemie, online article
Eukaryotes and most prokaryotes require isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) as biosynthetic precursors of terpenes. Whereas animals generate these essential metabolites via the mevalonate pathway,[1] many human pathogens including Plasmodium falciparum and Mycobacterium tuberculosis are known to use the more recently identified non-mevalonate pathway, which is a potential target for drug development.[2–4] The final step of this pathway is catalyzed by IspH protein, which generates a mixture of IPP and DMAPP by reductive dehydration of 1- hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate (HMBPP, Figure 1a).[5–11] Recently, Rekittke et al. described the first X-ray structure of IspH protein from the hyperthermophilic eubacterium Aquifex aeolicus in its open state.[12] Herein, we report the crystal structure of the IspH protein from Escherichia coli[11] in its closed conformation, which serves as basis for a detailed discussion of the catalytic pathway