The Menagerie of Human Lipocalins: A Natural Protein Scaffold for Molecular Recognition of Physiological Compounds
10-Mar-2015
Acc. Chem. Res., 48 (4), pp 976–985, DOI: 10.1021/ar5003973
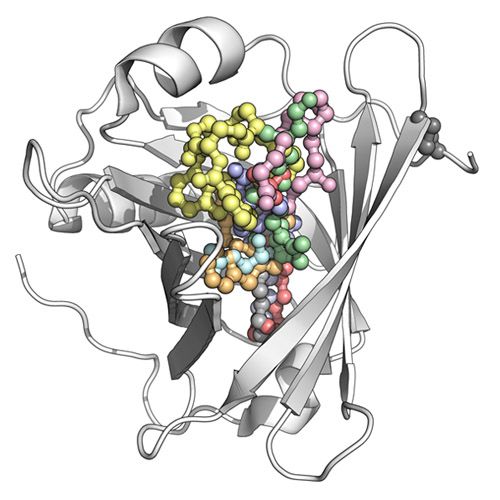
While immunoglobulins are well-known for their characteristic ability to bind macromolecular antigens (i.e., as antibodies during an immune response), the lipocalins constitute a family of proteins whose role is the complexation of small molecules for various physiological processes. In fact, a number of low-molecular-weight substances in multicellular organisms show poor solubility, are prone to chemical decomposition, or play a pathophysiological role and thus require specific binding proteins for transport through body fluids, storage, or sequestration. In many cases, lipocalins are involved in such tasks. Lipocalins are small, usually monomeric proteins with 150–180 residues and diameters of approximately 40 Å, adopting a compact fold that is dominated by a central eight-stranded up-and-down β-barrel. At the amino-terminal end, this core is flanked by a coiled polypeptide segment, while its carboxy-terminal end is followed by an α-helix that leans against the β-barrel as well as an amino acid stretch in a more-or-less extended conformation, which finally is fixed by a disulfide bond. Within the β-barrel, the antiparallel strands (designated A to H) are arranged in a (+1)7 topology and wind around a central axis in a right-handed manner such that part of strand A is hydrogen-bonded to strand H again. Whereas the lower region of the β-barrel is closed by short loops and densely packed hydrophobic side chains, including many aromatic residues, the upper end is usually open to solvent. There, four long loops, each connecting one pair of β-strands, together form the entrance to a cup-shaped cavity. Depending on the individual structure of a lipocalin, and especially on the lengths and amino acid sequences of its four loops, this pocket can accommodate chemical ligands of various sizes and shapes, including lipids, steroids, and other chemical hormones as well as secondary metabolites such as vitamins, cofactors, or odorants. While lipocalins are ubiquitous in all higher organisms, physiologically important members of this family have long been known in the human body, for example with the plasma retinol-binding protein that serves for the transport of vitamin A. This prototypic human lipocalin was the first for which a crystal structure was solved. Notably, several other lipocalins were discovered and assigned to this protein class before the term itself became familiar, which explains their diverse names in the scientific literature. To date, up to 15 distinct members of the lipocalin family have been characterized in humans, and during the last two decades the three-dimensional structures of a dozen major subtypes have been elucidated. This Account presents a comprehensive overview of the human lipocalins, revealing common structural principles but also deviations that explain individual functional features. Taking advantage of modern methods for combinatorial protein design, lipocalins have also been employed as scaffolds for the construction of artifical binding proteins with novel ligand specificities, so-called Anticalins, hence opening perspectives as a new class of biopharmaceuticals for medical therapy.